"A balanced approach to nutrition"
“a deficiency of any single nutrient is enough to limit yield”
– Justus von Liebig This simple “Law of the Minimum” is visually illustrated in Liebig’s barrel analogy below.
This simple “Law of the Minimum” is visually illustrated in Liebig’s barrel analogy below.
By carrying out broad spectrum soil analysis and learning how to correctly identify deficiencies in crops, remedial action can be taken to mitigate the effects and, perhaps more importantly, preventative action can be taken in the future to try to ensure the crops in similar situations are not undersupplied again.
“Law of the Maximum” – whereby an element that is in excess might be tying up an element that you want. for example, it is a well know fact that if you over lime you will tie up most of the elements in the soil, making them unavailable to the crop.
Calcium, magnesium and iron are the 3 most dominant elements found in UK soils, and some soils contain all 3 in excessive amounts. They are all very good at tying up phosphate regardless of soil index.
Although it is well documented that nitrogen is the key driver of outright yield for most crops, the supporting nutrients, in particular phosphate, potash, sulphur and magnesium, are equally as important to provide a fertile soil in which the response to N fertiliser can be maximised, and costly losses to the environment minimised.
Secondary nutrients are only termed thus due to the fact that they are required in slightly smaller quantities than N, P & K, they are not secondary in importance to the crop. Similarly trace elements are only required in tiny quantities but the effect of deficiency can be devastating. A balanced approach therefore provides additional nutrients which may be limiting the response of the NPK fertiliser applied.
Nutrient interactions in the soil
By using Mulder’s Chart to illustrate the interactions of soil nutrients, we can demonstrate how a high level of one nutrient can antagonise the availability of another (e.g. potassium – see table below), whilst the increased levels of another nutrient can have a synergism with one another, increasing their availability (e.g. Calcium and Phosphate).
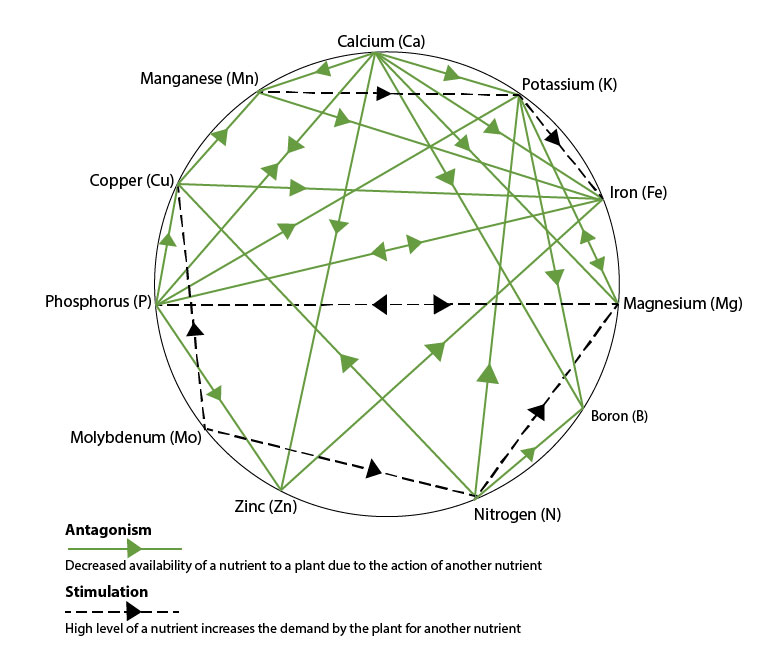
"Addressing the balance of nutrient availability"
In understanding the nutrient interactions enables us to give professional advice on how to address the nutritional balance, through product choice and increased nutitional availability from the soil reserves.
Guidelines for Calculation of Phosphate, Potash and Magnesium Removal by Crops
| * ** *** | Offtake value is per tonne of grain or seed removed but includes nutrients in straw when this is also removed and weight is unknown. The magnesium offtakes are based on very limited data and are for guidance only. n/d = no data. Use these values when straw weight is known. Potash content of straw can vary considerably – higher than average rainfall between crop maturity and straw baling will reduce straw potash content. Information on non-cereal straws is limited so values should be used only as a guide. |
Improving soils with low nutrient status
Soil analysis measures the average nutrient concentration in the depth of soil sampled; theoretically a concentration of 1mg/l to a depth of 10cm in soil represents 1 kg/ha of (elemental) nutrient. In practice however, plants also obtain nutrients from below sampling depth and from the slowly available pool that is not measured by analysis (see nutrients in the soil diagram in soil sampling section). This often gives rise to some confusion especially as these sources are very variable depending on soil type and past fertilisation and manuring. It is therefore possible to have a soil analysed at 125mg/l of K (Index 2) which supplies a cereal crop with peak uptake of 250kg/ha K (300kg/ha K2O).
Conversely the addition of 100kg/ha of phosphorous, potassium or magnesium (in fertiliser terms this represents 230kg P2O5, 120kg K2O and 170kg MgO) will not result in an increase of 100mg/l of readily plant-available soil P,K or Mg. This is because some of the nutrient applied will remain in the readily available pool measured by analysis. Unfortunately the proportion remaining available and thus affecting soil analysis values differ widely with different soils and conditions and is not reliably predictable.
With present knowledge it is not possible to provide more than a very rough guide as to how soil values will change with additions (or removals) of nutrient. The following figures have been suggested but it is likely that the range in practise is even wider than indicated
To increase soil Olsen P by 10mg/l requires approximately 400-600kg/ha P2O5
To increase plant-available soil K by 50mg/l requires apporximately 300-500kg/ha K2O
More potash will be required on heavier soils where the clay type can make a diffference. Where either the P or K status needs to be increased, ash based, slow release fertilisers – like p-grow, fibrophos or upkeep – are respectively cost-effective nutrient sources.
NPK Online Nutrient Calculator
Follow this link below to calculate your individual field and crop nutritional requirements using DEFRA’s online version of the RB209 Fertiliser Manual.
Helping you with the 3R’s, in selecting the “Right Product, of the Right Balance, for the Right Job” as supplied by JSE-Systems.
Click here
Nutrient List
Here is some background information on all of the main plant nutrients and the specific importance of each nutrient to crops and how you can manage the nutrient balance.
 Phosphorus in the soil
Phosphorus in the soil
The total phosphorus content of soils is generally high. However, only a fraction of it is available directly to the plant and the majority is adsorbed to the soil.
Phosphate dynamics in soils
The availability of phosphorus can be categorised as follows:
- soluble state (directly available to the plant), such as:
~ Orthophosphate in the form of H2PO4- and HPO42-.
- unstable state (available after mobilisation), such as:
~ P fraction which has been adsorbed onto oxides and hydroxides of iron or aluminium as well as clay mineral.
~ calcium-, magnesium-, potassium-, sodium- and ammonium phosphate depending on the concentration of cations in the soil solution.
~ easily soluble organically bound phosphorus.
- stable state (difficult or often not at all available to the plant) such as:
~ calcium-, iron- and aluminium phosphate (inorganic)
- Phytate (organic).
Effect of pH on P availability:
The availability of phosphorus in the soil depends largely on the pH value. The greatest mobilisation occurs at a pH value between 6 and 7. The danger of phosphorus fixation is greater with an increasing soil pH. However, the availability can be improved at a relatively high pH (7.5-8) through addition of organic matter and at a high pH (>8) from addition of S or gypsum.
Increasing acidity of the soil results in the development of aluminium and iron phosphate. The availability of phosphorus can be improved by liming of the soil.
Effect of phosphorus fertilisation on the soil:
- Creation of stable soil crumbs and improved structure
- Proliferation of micro-organisms in the soil and support of their activity
- Increased humus content as a result of greater root growth
Soil fertility status
The fraction of phosphorus that is easily taken up from the soil solution is the important fraction for plant nutrition. Analysing soil for its plant available nutrients is a useful tool in calculating fertiliser requirements. Most countries have a scale of available P in soils and different crops require different P levels according to the responsiveness of that crop to P. It is important that P is neither limiting nor in excess since an excess of P not only increases the risk of leaching into the environment but also can cause problems with micronutrient availability.
Phosphorus in the plant
P is mainly taken up from the soil solution in the orthophosphorus form by the root hairs. These hairs are also able to solubilise a proportion of the unstable phosphate fraction through the excretion of acids. Therefore, a well developed root system is essential for the uptake of phosphorus.
Phosphorus is irreplaceable as a main nutrient for the plant. It is the constituent part of many plant compounds and affects the entire plant metabolism.
Functions of phosphorus in the plant:
- Important for the transfer of chemically bound energy in various processes in the plant metabolism.
- Central function in synthesis, breakdown and conversion of fat, proteins, carbohydrates and vitamins.
- Important component of biological membranes.
- Supports root and shoot growth of crops.
- High demand of phosphorus during ear development and flowering and for the development of fruits and seeds (development of phytin as a P reservoir for germination).
- Improves processing characteristics and the contents of bio-active substances in food crops. the practical value and the biological value of the products.
Phosphorus deficiency symptoms
- Plants are small and show a stunted, upright growth habit with rigid leaves.
- Root growth, as well as shoot growth (particularly in cereals) is reduced.
- Flowering and ripening are delayed.
- The energy transfer system does not proceed as normal and therefore the entire plant metabolism is comprimised
- Initially the older leaves may darken in colour, later often turning red and eventually dying. The cause of this is the accumulation of chlorophyll and an increased anthocyanin content
- Often older leaves are dropped prematurely.
- Plants may become less resistant to frost.
 Potassium fractions in the soil
Potassium fractions in the soil
The concentration of potassium in soils ranges from around 0.3 to 3%. This amount is almost exclusively present in an inorganic form.
The dynamic exchange processes of potassium in soils are represented schematically in the potassium dynamics in soils diagram opposite.
Soil fertility status
The fraction of potassium that is easily available from the soil solution is the important fraction for plant nutrition. Soil analysis for its plant available nutrients can be used to calculate the fertiliser requirements.
Most countries have a scale of K index with a recommended value for the different types of crop depending on their K requirement. These indices are also greatly affected by the soil.
Potassium in plants
Potassium is taken up from the soil solution by plants only as potassium ions. It is very mobile in the plant and is vital for the plant as it affects in many ways the various metabolic processes.
Functions of potassium in plants:
- Affects photosynthesis directly through an effect on the chloroplasts and indirectly through an effect on the closing mechanism of stomata.
- Involved in the metabolism of plants through its involvement in activating more than 50 enzymes.
- Improves the efficiency of water utilisation and thereby reduces drought stress.
- Improves synthesis of carbohydrates such as sugar and starch.
- Supports transport and storage of carbohydrates from the leaves to the storage organs (tubers, grains, beets etc.).
- Improves the qualitative value of products through higher protein and vitamin content.
- Positively increases the content of organic anions and improves, mainly in connection with sulphate ions, the taste of fruit and vegetables.
- Enhances the development of supporting tissue. This reduces lodging (i.e. in cereals) and supports a vigorous bushy growth in broad-leaved crops for maximum interception of sunlight
- Increases the natural resistance of plants against disease, insects and frost.
- Results in a decrease of black spot in potatoes.
- Initially visible on older leaves as K is easily transported via the phloem.
- Leaves become flaccid and droop.
- The entire plant looks limp and wilted
- Starting from the margin leaves become increasingly bright green.
- Later, necrotic patches develop on the margins and leaf tips
- Delayed growth occurs.
- Leaves stay small and fruits remain tightly attached to the plant.
- The decreased lignification of cell walls increases the risk of lodging in cereals and the susceptibility to fungal infections.
 Magnesium dynamics in the soil
Magnesium dynamics in the soil
Besides the Mg2+ ions occurring in the soil solution, magnesium is either adsorbed to cation exchangers such as organic matter or clay particles in the exchangeable fraction or it is bound inside the crystals of soil silicates. Only the first two fractions are available to plants.
The strength with which the Mg ions are bound to the exchange surfaces is relatively low because of the large hydrate sphere of the magnesium ion. This results in an increased risk of leaching, especially on soils with a low Cation Exchange Capacity (CEC) coupled with a low pH.
- Plant-available Magnesium derived from the weathering of silicates is made available only very slowly over geological timescales
- Magnesium is present in some soils as magnesites and dolomites. At pH values >6, this magnesium is largely insoluble and therefore unavailable
- Many soil types are inherently low in Magnesium. Light textured and acidic soils are often Mg depleted and the supply is often insufficient for many agricultural and horticultural crops.
- The uptake of Mg by the plant is negatively affected by large K:Mg and large Ca:Mg ratios as well as a low pH. This means that even at high magnesium concentrations in the soil a latent or even severe deficiency of magnesium in plants is possible.
Supply in the soil
The fraction of magnesium easily available from the soil solution is important for the nutrition of plants. Soil analysis can reveal the current supply in the soil and allow the calculation of required fertilisation. Most countries have a scale of Mg index with a recommended value for the different types of crop depending on their Mg requirement. These indices are also greatly affected by the soil.
Magnesium in the plant
Plants take up magnesium from the soil solution as Mg2+ ions. Mg is highly mobile in the plant and is important for the correct functioning of many important metabolic pathways.
Functions of magnesium in the plant
- central atom of the chlorophyll molecule and therefore essential for the light dependant reaction of photosynthesis.
- essential for synthesis, transport and storage of important plant substances such as carbohydrates, proteins and fats.
- magnesium activates more enzymes than any other plant nutrient
- regulates the energy balance of plants, as it is important for facilitating reactions between enzymes and ATP, the energy currency in plants.
- effects RNA synthesis and therefore the translation of genetic information into proteins.
- component of pectin, important for stability of cells and phytin, an energy rich phosphate store hugely important for seed germination
- integrated part of ribosomes and the cell matrix as well as aiding stabilisation of cell membranes.
- is required for cell wall synthesis.
- has hydrating characteristics, and therefore affects water balance and effectiveness of enzymes.
- Magnesium and manganese increase the concentration of valuable components such as citric acid and vitamin C. They increase the nutitional quality of frozen vegetables and the resistance of potatoes against discoloration during processing to mash and potato powder.
Magnesium deficiency symptoms
- Deficiency symptoms first occur on older leaves as chlorotic spots between leaf veins.
- necrosis and red discoloration of stems occur during prolonged periods of deficiency
- the entire plant looks wilted and limp during intense sun radiation, similar to wilting seen as a result of K deficiency. This relates to an imbalance of water in the plant. Single leaves look stiff and brittle.
- Chlorophyll content and the number of chloroplasts in the plant are decreased.
 Sulphur in the soil
Sulphur in the soil
The sulphur content of soils varies widely, in humid climates, S concentration is typically around 0.02-2 %, moorland soils may contain 1 % and in marshland, S concentration can be as high as around 3.5 %.
Sulphur may occur in the soil inorganically or organically bound. Depending on the state of the soil, the inorganically bound sulphur can occur as elementary sulphur or in different forms of oxidation (sulphide, sulphate, thiosulfite etc.). Sulphur containing organic compounds are: amino acids, proteins, polypeptides and others.
Deficiency symptoms
- Deficiency symptoms often occur first on younger leaves, (cf. N deficiency which tends to appear on older leaves first).
- Large areas of generalised chlorosis appear on the leaves.
- The whole plant appears rigid and brittle.

- Typical symptoms in oilseed rape are stunted growth, spoon-like arched leaves, pale yellow or white petals and pods can appear bladder-like and bloated.
- Until the 80’s, SO2-emissions of industrial origin were generally sufficient to supply the complete S requirement of most crops.
- Several measures for lowering industrial emissions for cleaner air have resulted in a significant decrease of atmospheric sulphur emissions. This in turn has resulted in a decrease in S depositions which have now decreased to pre-industrial levels.
- A huge deficit in sulphur is predicted especially for Asia, but also for other regions of the world.
- The input of sulphur in rural areas far from industrialisation reaches only an average of 5-10 kg S ha-1. This does not cover the requirements, which depending on the crop and yield level, ranges between 5-80 kg S ha-1.
 In many areas of the world, shortage of sulphur has now become the single largest limiting factor restricting plant productivity.
In many areas of the world, shortage of sulphur has now become the single largest limiting factor restricting plant productivity.- Many crops, such as oilseeds, legumes, onions, leek, and garlic, require S for the production of aromatic flavour compounds. For these crops in particular, fertilisation with sulphur frequently results in enormous increases in yield and quality.
- In addition, the supply of sulphur through manure and other farm wastes is often insufficient. In the first year only 5-10 % of the complex sulphur is available for the plant. For crops that have an early sulphur requirement such as oilseed rape and cereals, use of such manures over several years is still not sufficient and therefore a mineral fertilization is recommended.
Manganese in the soil
Manganese occurs mainly in oxide form but also occurs in silicates. Mn2+ ions are released into the soil solution during weathering of silicates. As well as the clay content of the soil, the pH and the redox potential of the soil are also important factors determining the potential of a soil to hold such readily exchangeable manganese.
With a decreasing pH value and decreasing redox potential comes an increase in the concentration of plant available Mn ions. A low redox potential occurs during low oxygen concentrations in the soil i.e. compaction, flooding, standing water). In contrast, a high pH value and adequate soil aeration decreases the concentration of Mn-ions.
Manganese deficiency mainly occurs in organic and carbonate rich soils, high pH soils and very light sandly soils which are often well aerated because of manganese fixation. Humus rich and podsol sandy soils are rather Mn poor as the manganese is less fixed.
Manganese in the plant
Manganese is taken up by the plant only as Mn2+-ions. This process can be inhibited by high concentrations of Mg2+-, Ca2+-, Cu2+- and iron ions. Manganese either stimulates, or is a component of many enzymes and, therefore, greatly affects the metabolism of the plant.
Functions of manganese in the plant:
- directly affects photosynthesis by assisting the synthesis of chloroplasts.
- Important role in synthesis of fatty acids.
- Affects energy budget by regulating carbohydrate metabolism.
- Reduction of nitrates in plants is only possible if sufficient manganese is present.
- Increases growth of secondary roots.
- Stimulates growth due to effect on elongation of cells.
- Similar to copper, manganese is important for immobilisation of free oxygen radicals.
- Manganese and magnesium increase the concentration of valuable ingredients such as citric acid and vitamin C. They increase the quality of frozen vegetables and the resistance of potatoes against discoloration during processing to mash and potato powder.
Manganese deficiency
- youngest and mid leaves show chlorotic spots between the veins because the development of chloroplasts is negatively affected.
- Gramineae show chlorotic and necrotic strips.
- especially characteristic are the deficiency symptoms in oats which are called: grey speck or early blight; here the plant exhibits dirty grey strips or spots on the base of the leaves. The entire water balance is affected.
- Manganese deficient plants have a lower cell volume. Cell elongation and growth of secondary roots are negatively affected.
Manganese toxicity
- occurs on acidic soils because these soil solutions mainly contain Mn2+ ions which are easily taken up.
- older leaves, leaf bases and stems show black-brown spots as a consequence of MnO2 deposits. Later on they show chlorotic margins.
- Induced deficiency of iron, magnesium and/or calcium can occur and add to the symptoms exhibited by the plant.
Sodium in the soil
- Sodium occurs only in a compound form in soils, predominantly as salts.
- Sodium does adsorb onto clay minerals but the bond is weaker than that of potassium ions and therefore sodium has a higher propensity to be leached Therefore in areas of high rainfall such as trpicall or sub-troical climates, soils are generally depleted in sodium which is washed down into deeper soil layers.
- In contrast, in arid or semi-arid areas an accumulation of Na in the top soil frequently occurs because the rate of evaporation exceeds the replacement of water from the soil. This often results in a deterioration of the soil structure which has a negative effect on the water and air balance of the soil. In addition, the pH becomes more alkaline with an increasing Na content.
Sodium in the plant
- Even though sodium plays a less important role in plant nutrition than potassium or magnesium, the positive effects of sodium fertilisation on yield and quality of sodium loving crops (e.g. Chenopodiaceae) are clearly evident. Sugarbeet as the most important crop species in this group is well known example which has a relatively high sodium requirement. Sodium supports the synthesis of glucose and its conversion to fructose which is stored in the beet.
- Sodium controls osmotic pressure in plant cells and results in a more efficient use of water.
- Na ions can often substitute K ions in some metabolic and osmoregulatory functions and therefore the two nutrients are interchangeable to varying degrees according to plant group.
- Sodium is important for some C4 plants (e.g.. amaranth) for CO2 uptake.
Sodium in animals
- In animal nutrition, a sufficient supply of sodium is an important factor in maintaining the productivity of the animals. Sodium deficiency results in loss of appetite, decreased milk production, weight loss and has implications for health and fertility of the animals.
- Approximately 2g Na kg-1 DM in the basic ration is necessary to satisfy the daily requirement for Na in dairy cattle. Field studies have demonstrated that in many regions the average sodium content of grassland is just 0.1-1g Na kg-1 DM and therefore clearly below the required amount.
- Sodium containing fertilisers such as Magnesia-Kainit have been successfully used for over a century for greatly increasing the sodium content of grassland and can guarantee meeting the Na demands of the animals.
- Studies and trials have demonstrated that besides an improved Na supply from the nutritional point of view, the increased sodium also has a major effect on palatability of forage which can increase dry matter intakes by around 10%.
Boron in the soil
The boron content of soils in humid climates ranges between 5-80 mg kg-1. Soils rich in sand typically contain a lower boron content (5-20 mg kg-1) than soils rich in clay and organic matter (typically 30-80 mg kg-1). In saline soils, boron concentration may be so high that it can reach levels that are toxic to plants. Boron is present in the soil solution in the form of boric acid (H3BO3) which is produced during weathering of mica and tourmaline. Boric acid dissociates above pH 6.3 and the negative charge of the anion produced,is attracted to the positive surfaces of iron and aluminium oxide, clay minerals and organic substances thus limiting availability to the plant. Since boron is taken up with the soil water, boron deficiency mainly occurs during dry periods.
Boron in the plant
Boron belongs to the group of essential micro-nutrients and affects many processes in the plant metabolism. The requirement of the various crops for boron is very different. For example, monocotyledonous plants such as cereals generally have a lower requirement for boron than dicotyledonous crops. This is thought to be due to key differences in the cell wall structures of the two groups. Boron is taken up by plants mainly in the form of boric acid.
Functions of boron in the plant
- Promotes synthesis of structural carbohydrates in the cell wall.
- Improves stability and function of cell membranes.
- Enhances sucrose synthesis and transport of assimilates to storage organs.
- Regulates RNA synthesis, which in turn affects synthesis of nucleic acids and therefore proteins.
- Supports plant growth by stimulating cell division.
Boron deficiency symptoms
- Deficiency symptoms are visible first on the youngest growing points.
- Subsequently, roots and shoot tips die and young leaves wilt probably as a result of an inadequate supply of assimilates as well as the interrupted water supply.
- Next the growth of side shoots is increased because of the lack of apical dominance.
- Flower formation and fertilization are affected.
- Transpiration is increased and the water balance is negatively affected.
- Rhizobia development in the roots of legumes is inhibited.
- Typical deficiency symptoms in beet and chard include brown heart and dry rot. In alfalfa, tip yellowing is commonly seen.
Boron surplus in the plant
- Yellowing of older leaves which results in necrotic and perforated tissue.
- Cucumbers and legumes are very susceptible as the range between adequate supply and toxicity is very narrow.
Copper in the soil
The copper content of unpolluted soils typically ranges between 2-40 mg Cu kg-1 soil. Copper has a tendency to bind to the soil organic matter of the soil. It is adsorbed by manganese and iron oxides or it can be bound to the lattice silicates. In addition, it can precipitate as the hydroxide, carbonate or phosphate form. The concentration of copper in the soil solution depends on the pH value and the available chelating agents. The proportion of exchangeable copper generally increases with decreasing pH. Copper deficiency occurs on recently cultivated moorland soils and due to Cu fixation also in podsol soils rich in organic matter.
Copper in the plant
Plants take up Cu2+-ions freely from the soil solution or as soluble copper complexes. As a component of several enzymes, copper has a positive effect on the plant metabolism.
Function of copper in the plant
- Regulates the photosynthetic electron transport.
- Similar to manganese, copper assists in the binding of free oxygen radicals which renders them innocuous.
- Important for sub processes of lignification.
- Important for rhizobia production associated with legumes.
Copper deficiency
- In cereals, the youngest leaves turn white due to damaged chloroplasts.
- Leaves roll together like a thread, wilt and eventually die.
- The internodes are shortened.
- Ears or panicles develop poorly with many blind grain sites.
- in fruit trees, growing points are stunted and both flowering and fruit set are hampered.
- Copper deficiency can be excaserbated by an increased nitrogen supply since copper forms strong bonds with the amino acids produced.
- Copper is often bound to the organic matter in newly cultivated fields and can also be locked up after heavy liming on reclaimed marshland or boggy areas.
Copper excess
- Only generally occurs at pH values of < 5.
- Causes yellowing of the youngest leaves.
- Can induce iron, zinc and molybdenum deficiency in plants.
- Can accumulate in the plant roots and result in damage the root cell membranes.
- Greatly alters enzymic activity which inhibits root growth.
- Plants exhibit different grades of copper tolerance. This is based on their ability to take up less copper from the soil solution or via a mechanism which excretes excessive copper or to transforms it into a harmless form.
Zinc in the soil
The zinc content of unpolluted soils ranges between 10 – 80 mg kg-1 and the Zn content of sandy soils is generally lower than that of loamy soils. Freely available zinc in the soil solution binds mainly to the organic matter in the soil. In addition, it can be found adsorbed onto iron, manganese and aluminium oxides or strongly bound to the lattice of clay minerals and silicates. Additional immobilisation of zinc occurs when the sulphate and phosphate content in the soil solution are excessive. The availability of zinc is strongly affected by the pH and the total Zinc content of the soil. The proportion of exchangeable Zinc decreases with increasing pH and is already greatly reduced at pH 6. With increasing pH, the affinity of zinc to manganese oxide and iron oxide increases strongly. Under anaerobic conditions, zinc can be precipitated into the barely soluble sulphide form which is largely unavailable to plants Zinc can be leached from the soil but this process generally only occurs in acidic soils.
Zinc in the plant
Zinc is taken up by plants from the soil solution either as the Zn2+ ion (at low pH) or as the zinc hydroxide ion (at higher pH values). Plants grown in acid conditions of less than pH 6 are rarely short of Zinc since the availability under such conditions increases greatly. Zinc activates or is a component of several enzymes and therefore affects many metabolic processes in the plant.
Functions of zinc in the plant:
- As an essential component of RNA polymerase which catalyzes RNA synthesis, which in turn affects production of proteins.
- As a component of enzymes, zinc catalyzes the synthesis of fructose-6-phosphate, an important metabolite in glycolysis and therefore photosynthesis.
- Is essential for the stability of ribosomes.
- Affects the indole-3-acidic acid content which is important for regulation of plant growth.
Zinc deficiency symptoms
- Leaves are small and their tips are often white. The entire plant is often stunted (dwarfism).
- In fruit crops, ‘rosette’ or ‘little leaf’ development occurs because of jammed internodes. The growth of sprigs is inhibited and young shoots die. Premature leaf senescence can also occur.
- Grape vines develop an increased number of Geiztrieben and the grapes stay small.
- Older and middle leaves display chlorotic spots with necrotic areas.
Zinc toxicity
An excess of Zinc can be toxic in plants although the tolerance levels are usually high. Some plants are able to store surplus zinc in their vacuoles. Zinc toxicity results in:
- Inhibition of root development.
- Chlorosis seen on the younger leaves.
- Induced iron deficiency.

