Agricultural Lime - pH Decline
Interpretation of soil pH
 The degree of soil acidity or alkalinity is measured by what is known as the pH scale.
The degree of soil acidity or alkalinity is measured by what is known as the pH scale.
A figure of pH 7 represents a materials relationship to the neutral position of pure water at pH 7.0. Figures below 7 indicate increasing acidity and above 7 increasing alkalinity. The optimum for general cropping is between pH 6.5 and 7.0 (5.8 on peaty soils). For permanent grassland the optimum pH is slightly lower between 6.0 and 6.5 (5.3 on peaty soils).
The soil pH is the measure of the hydrogen ions in the soil and is what determines how acid or alkaline the soil is. To cure an acidic soil a recommendation of aglime is required which is produced once a pH soil sample has been taken and mapped on each field. For information on how this is done please see below or click here.
In-field measurements using pH indicator on some soils where free chalk or lime particles exist may give lower values than laboratory results for the same field. This is because grinding the soil for laboratory analysis pulverises any chalk/lime particles and the pH as measured is increased.
Unless steps are taken to redress the balance of soils by applying a liming material there will be a natural reduction in the lime status of most soils. This results in a natural increase in acidity and in many cases a reduction in soil fertility and damage to soil structure.
Over the past 2 decades a decline in lime application has resulted in an increase in the proportion of UK soil samples exhibiting pH values below optimum levels. This means that the full agricultural potential for these soils has not been realised.
Although, ‘The British Survey of Fertiliser Practice’* suggests that the steady decline in agricultural land area receiving lime in Britain appears to be reversing.
However, the survey also concludes that this area is still considerably less than that calculated to require liming. In addition, it states the principle causes of acidification have not lessened, and it is reasonable to believe that significant areas of arable land, and more particularly grassland, are at a pH level which could limit productivity.
*Organised and jointly funded by the Fertiliser Manufacturers’ Association (FMA), the Department for Environment, Food and Rural Affairs (DEFRA) and the Scottish Executive Rural Affairs.
Influence of soil pH on plant nutrient availability & plant growth
Acidity below pH 6.0 will reduce the availability of some nutrients, especially phosphorous.
Availability of trace elements is radically affected by pH and the need for trace elements should be assessed only after any required amendment of acidity has been undertaken and has had time to take effect.
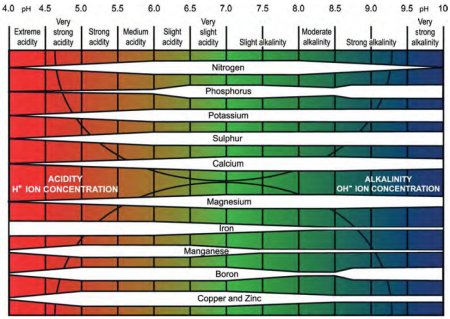
The availability of different nutrients at the different pH bands is indicated by the width of the white bar: the wider the bar, the more available is the nutrient (redrawn for PDA from Truog, E. (1946). Soil reaction influence on availability of plant nutrients. Soil Science Society of America Proceedings 11, 305-308.).
Agricultural Lime - Losses
LEACHING | CROPPING | FERTILISING | POLLUTION
AVERAGE LIME LOSSES CAN AMOUNT TO 1250 Kg CALCIUM CARBONATE PER HECTARE PER YEAR
With Intensive arable and livestock rotations, there is a natural reduction in the lime status and pH of the soil, with lime losses occurring from:
Cropping –
- Organic and inorganic acids formed during decomposition of crop residues (acid forming)
- Plants remove calcium and magnesium from the soil (acid forming)
- Deep tillage may bring acid subsoil into the root zone (acid forming)
- Plants release hydrogen ions (H+) to the soil (acid forming)
Leaching – Calcium and magnesium carried out of the soil root zone by downward movement of soil water, often with Nitrogen (NO3)
Fertilising – Ammonium nitrate fertilisers aid soil acidity by nitrification of NH4 – Nitrogen to Nitrate which releases hydrogen ions.
Pollution – The products of fossil fuel combustion from power plants, industry and motor vehicles are returned to earth in rainfall as nitric and sulphuric acid.
Sampling for assessment of agricultural lime requirements
Soil samples should be taken methodically from a number of places in the field and tested individually since acidity frequently occurs in patches in the field. Test results should be plotted on a field map so that any lime required may be applied in the right place and at the correct and most economic rate. Although poor and patchy crop performance and the presence of acid loving weed species are rough indications of lime deficiency, the acid reaction to indicator solution or pH meter is the only reliable method of assessing lime requirements.
The use of precision GPS technologies will help make the mapping of sites more accurate and potentially save time. The application of materials will then become more precise and cost effective.
Liming recommendations & product selection
Liming recommendations are carried out by our field advisor’s on a field to field bases once each field has been sampled. Once the liming agent has been chosen from various different grades of limestone or chalk based Aglime products from our various supply quarries, including our own or from the sugar refinery processing products; such as LimeX70, or recycled industrial process; such as potassic lime, an accurate recommendation can be produced for the specific crop (or rotation) from the neutralising value of the product and delivery and application can be arranged.
Aglime supply & application
Aglime, calcium carbonate (CaCO3), is sourced from local, quality sources to keep haulage cost to a minimum and is available in a range of sizes from dust free course granular lime to fine powder lime. The consistent quality of Aglime products gives advantages in a number of applications.
JSE-Systems have access to 7 lime spreaders across East Midlands, operated by higly experienced operators to ensure the highest quality of spreading is achieved and at a time when it suits you. We operate modern, well maintained equipment fitted with the latest GPS technology to assits in application and offer variable rate applications. Our local knowledge of harvesting throughout the region means we can make sure that lime is spread to land as convenient as possible to your harvesting, cultivation and drilling/planting plans.
- For more information on ALA lime recommendations according to soil type and cropping click here
- For more information on ALA optimium pH for crop growth click here

